Graphene – wonder material or curious nonentity? – Ms Egan-Smith
You may have heard of graphene – first isolated in 2004 by Professors Andre Geim and Kostya Novoselov working at the University of Manchester. You will almost certainly have heard of graphite, and may even have a tennis racket that contains graphite. What is the connection? Put simply, graphene is a single layer of graphite i.e. a single sheet of carbon atoms arranged in a perfect hexagonal lattice; many of layers of graphene together make graphite.
Graphite is also found in many pencils and there is a chance that you may even have made graphene if you have ever drawn with a pencil.
Graphene has been hailed as a wonder material as its properties are so impressive:
- It is incredibly strong – it has the highest tensile strength of any material [i.e. the force needed to pull it so that it breaks] and is about 200 times stronger than steel
- At only one atom thick, it is the thinnest material in the world – only 0.34 nm thick or about million times thinner than a human hair
- It is incredibly light – 1 square metre of a graphene film weighs only 0.38 mg. In other words a sheet of graphene weighing the same as a fun-sized Mars bar would cover 8 football pitches.
- It is very flexible
- Is the best conductor of electricity known
- Is totally impermeable to even the smallest atom [helium]
- Is chemically very inert [in its pure form]
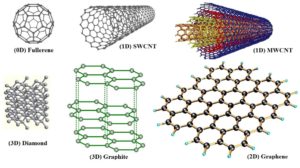
Why does graphene have such fantastic properties? The reasons lie in its structure [a more detailed description of the bonding in graphene is given at the end]. Graphene is a true 2-dimensional material. At this point we need to rethink our understanding of the term as in this sense 2-D does not refer to something with only two dimensions [length and width, say]; after all, even though graphene is only one atom thick, it does have length, width and height. Rather it means that the substance can only be extended by adding atoms in two dimensions without altering its properties. In other words, we can have a sheet of graphene with any length and width and it will display the same properties. If we change the height – i.e. add atoms in that direction [so it is no longer just one atom thick] – we dramatically change the properties and make something else. With this way of defining structures, we see that other ‘traditional’ 3-D materials are in fact 1- or even 0-dimensional!
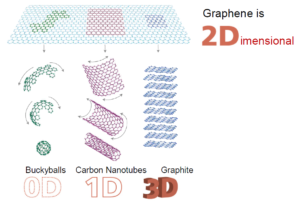
Image from: Graphene MOOC – www.graphene.manchester.ac.uk
The potential applications of graphene [and modified graphenes such as graphene oxide] are far-reaching and have generated huge excitement amongst the scientific community. Indeed, a National Graphene Institute was set up at the University of Manchester with the intention of generating an income from the applications it hopes to launch. Areas in which graphene could play a vital part include:
- hydrogels for energy storage to ease the problems of using fossil fuels [both limited supply and environmental damage]
- liquid crystal displays
- gas sensors
- polymer composites for increased strength with low density [similar to graphite composites currently used]
- surface coatings for touch screens and solar cells
- drug delivery, cancer treatment and regenerative medicine
- food packaging – its inertness and impermeability would prevent water or oxygen reaching the food causing it to spoil
- water treatment – graphene based membranes could make that easier in developing countries
- electronics that are faster and lighter
To get an overview of the applications being researched, visit http://www.graphene.manchester.ac.uk/explore/the-applications/
One problem with many of these is the need to prepare pure graphene on a large scale; currently this is not possible.
Millions of pounds have been spent researching graphene and its potential applications, yet twelve years later these applications remain largely theoretical. Will any of them be translated into a practical application that will justify the money spent – or will graphene turn out to be nothing more than an interesting, if ultimately useless, phenomenon?
The bonding in graphene [A Level +]:
The electronic structure of an isolated carbon atom is given by 1s2, 2s2, 2p2. When the atom bonds the 2s and 2p electrons will hybridise. The 2s orbital can be occupied by two electrons with opposing spins. The 2p subshell, however, consists of three different orbitals, 2px, 2py and 2pz which lead to an electron cloud oriented along the x, y, and z axis respectively. The hybridised atomic orbitals from which the molecular orbitals can thus be made up of different linear combinations of 2s and 2px, 2py and 2pz orbitals. Graphene undergoes sp2 hybridisation in which the hybrid orbitals are formed from the linear combinations of 2s, and 2px, and 2py electrons:
In graphene the electron in the 2pz orbital does not take part in the hybridisation; sp2 hybridised carbon therefore forms a layered structure in which the carbon atoms are joined by strong sigma bonds, which only occur in the xy plane, leading to the structure of graphene. The lowest energy configuration of the hybridised orbitals will be that in which the electrons are as far away from each other as possible. Therefore, angles of 120O between the different electron clouds, and hence between the different interatomic bonds in graphene, will occur.
A final note on the unhybridised electron in the 2pz orbital. These electrons form weak pi-bonds with neighbouring carbon atoms. [And in the case of graphite, a related sp2 hybridised structure, also the weak Van der Waals bond which hold the graphene layers together.] It is the electrons in the pi bonds which are responsible for the extremely high carrier mobility and the conductivity of graphene.
Novoselov and Geim received the 2010 Nobel Prize in Physics for their ground-breaking experiments on two dimensional graphene












Post Comment
You must be logged in to post a comment.